Foda na potassium iodate don maganin reagent na matakin abinci
Bayanin Samfurin
Sunan Samfura: Potassium iodate
Tsarkaka: 99.5%;
CAS:7758-05-6
MF: IKO3
MW:214
EINECS: 231-831-9
Wurin narkewa: 560 °
Bayyanar: Fari ko fari foda/ƙarfe mai ƙarfi
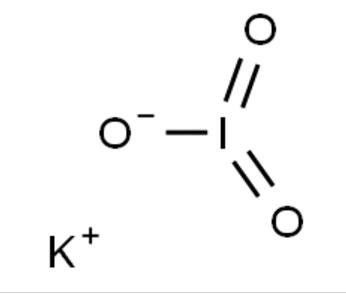
Potassium iodate gishiri ne mai yawan aidin tare da dabarar KIO3. A yanayi mai zafi da danshi, tururin aidin yana narkewa saboda yawan sinadarin potassium iodide. Idan aka kwatanta da potassium iodide, potassium iodate ya fi karko kuma yana da tsawon rai. Yana da ƙarfi wajen toshe shan iodine mai rediyoaktif ta glandar thyroid.
Kayayyakin Samfura
Potassium iodate foda ne mai launin monoclinic ko farin crystalline wanda ba shi da launi. Ba shi da wari. Yana narkewa a cikin ruwa, mai narkewar acid, ethylenediamine, ethanolamine, da potassium iodide ruwan sha; yana narkewa kaɗan a cikin ruwa sulfur dioxide; ba ya narkewa a cikin alcohols da ammonia.
Lu'ulu'u marasa launi ko farin foda; tsarin monoclinic; yawa 3.90 g/cm3; tsayayye a yanayin zafi na yau da kullun; yana narkewa a 560°C tare da wani ɓangare na ruɓewa, yana fitar da iskar oxygen; yana narkewa a matsakaici a cikin ruwan sanyi; 4.74 g/100mL a 0°C; yana narkewa mafi girma a cikin ruwan zãfi 32.3 g/100mL a 100°C; yana narkewa a cikin maganin potassium iodide; ba ya narkewa a cikin barasa da ammonia mai ruwa.
Aikace-aikace
1) Potassium Iodate tushen aidin ne da aka samar ta hanyar yin maganin aidin tare da potassium hydroxide. foda ne mai lu'ulu'u wanda ya fi iodide ƙarfi. Yana da narkewar gram 1 a cikin ruwa 15 ml. Ana amfani da shi azaman mai inganta kullu mai sauri; ana amfani da shi tare da potassium bromate azaman wakili mai hana iskar oxygen don canza furotin a cikin garin burodi wanda ke haɓaka girman burodi da siffarsa. Ana amfani da shi a cikin kayan gasa.
2) Potassium iodate wani sinadari ne mai ƙarfi wanda za a iya amfani da shi wajen gwajin wasu sinadarai na magunguna, misali: benzalkonium chloride, cetrimide, hydralazine hydrochloride, potassium iodide, phenylhydrazine hydrochloride, semicarbazide hydrochloride da makamantansu. A ƙarƙashin ma'aunin gwaji masu dacewa, iodate yana yin aiki da yawa tare da iodides da iodine. Duk da haka, abin sha'awa ne a lura a nan cewa ana iya yin titrations na iodate yadda ya kamata a gaban cikakken acid na halitta, barasa da sauran abubuwa na halitta.
Hanyoyin rage oxidation tare da potassium iodate koyaushe suna dogara ne akan samuwar iodine monochloride (ICl) a cikin wani maganin hydrochloric acid mai ƙarfi.
3) Ana iya ƙara aidin a cikin gishiri a cikin nau'in potassium iodide (KI) ko potassium iodate (KIO3). Saboda KIO3 yana da kwanciyar hankali mafi girma idan akwai gurɓataccen gishiri, danshi, da marufi mai ramuka, shine nau'in da aka ba da shawarar.
Potassium iodate, wanda aka samar a matsayin samfurin na biyu, ana rage shi ta hanyar amfani da carbon da aka kunna. Ana tsarkake samfurin ta hanyar lu'ulu'u daga ruwa. A madadin haka, ana iya magance baƙin ƙarfe (II) iodide, wanda aka shirya ta amfani da foda na ƙarfe da iodine, da potassium carbonate don samun potassium iodide. Ana iya shirya potassium iodide mai tsarki ta hanyar amsawar potassium bicarbonate tare da hydroiodic acid.
Bayanin Sufuri
Lambar Majalisar Dinkin Duniya: 1479
Aji na Hadari: 5.1
Rukunin Marufi: II
Lambar HS: 28299080
Ba da shawarar Samfuran
Ƙayyadewa
| Abu na bincike | Daidaitacce | Sakamakon bincike |
| Bayani | Foda fari ko mara fari/ƙarfe mai ƙarfi | Ya dace |
| Asarar bushewa% | ≤0.5% | 0.03% |
| Ni(%)≤ | 0.0019% | <0.002% |
| CLO3(%)≤ | 0.01% | <0.01% |
| SO4(%)≤ | Ya dace | Ya dace |
| Karfe mai nauyi (Pb)(%)≤ | <0.001% | <0.001% |
| PH | 5~8 | 6.0 |
| Kamar yadda(%)≤ | ≤0.0003% | <0.0003% |
| Gwaji | KIO3≥99.0% | 99.5% |








